Following assessment and approval by the European Medicines Agency (EMA), WHO has issued an emergency use listing (EUL) for Nuvaxovid on 21 December 2021. Earlier on 17 December 2021, the WHO had issued an emergency use listing (EUL) for Covovax.
Covovax and Nuvaxoid thus become the...
The recently completed UK-wide, ISARIC Study on analysis of 16749 patients with severe COVID-19 disease across 166 hospitals indicated that those with co-morbidity were at much higher risks while those with no significant comorbidity come out alive suggesting people...
One of unusual and most intriguing feature of heavily mutated Omicron variant is that it acquired all the mutations in a single burst in a very short span of time. The degree of change is so much so that some...
A bill H.R.2316 - Fire Fauci Act1 has been introduced into the US Senate to reduce Dr. Anthony Fauci salary along with audit for his correspondence and financial statements linked to COVID-19 outbreak. During the initial days of COVID-19 outbreak, in March...
In order to raise levels of protection across the population against the Omicron variant, the Joint Committee on Vaccination and Immunisation (JCVI)1 of UK has recommended that the booster programme should be expanded to include all remaining adults aged 18...
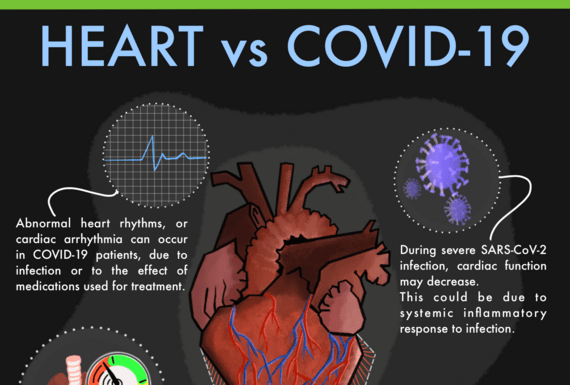
It is known that COVID-19 increases the risk of heart attack, stroke, and Long COVID but what was not known is whether the damage occurs because the virus infects the heart tissue itself, or due to systemic inflammation initiated...
To protect the NHS and save lives., National Lockdown has been put in place across the UK. People have been asked to stay home at home. This is in view of recent rapid increase in number of cases across...
A combination of biological and computational approach to study protein-protein interactions (PPIs) between the viral and the host proteins in order to identify and repurpose drugs for an effective treatment of COVID-19 and possibly other infections as well.
The usual...
Fluvoxamine is an inexpensive anti-depressant commonly used in mental healthcare. Evidence from the recently concluded clinical trial suggest that it can be repurposed to treat patients with COVID-19. It is found to reduce risk of severe COVID-19 symptoms, reduces need...
NeoCoV, a coronavirus strain related to MERS-CoV found in bats (NeoCoV is not a new variant of SARS-CoV-2, the human coronavirus strain responsible for COVID-19 pandemic) has been reported to be the first case of a MERS-CoV variant using ACE2....
There are overwhelming evidences to confirm that the dominant route of transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is airborne. This realisation has significant implications for fine-tuning of strategies to manage the pandemic, particularly in terms of importance...
Researchers at Southern Illinois University have reported a new variant of SARS COV-2 Virus in the USA.
According to reports published on preprint server which is yet to be peer-reviewed, researchers have identified a new variant using genomic virus surveillance approach.
Referred as 20C-US, this variant...
COVID-19 situation across Europe and central Asia is very serious. According to WHO, Europe could face over 2 million COVID-19 deaths by March 2022. Wearing masks, physical distancing and vaccination are key preventive actions that could help avoid reaching this...
Deltacron is not a new strain or variant but a case of co-infection with two variants of SARS-CoV-2. Over the past two years, different variants have emerged of the SARS CoV-2 strain with varying degree of infectivity and disease...
Coronaviruses and influenza viruses are sensitive to acidity of aerosol. pH-mediated rapid inactivation of coronaviruses is possible by enriching the indoor air with non-hazardous levels of nitric acid. Conversely, indoor air filter may unintentionally remove volatile acids thus prolonging...
Several new strains of the virus have emerged since the pandemic began. New variants were reported as early as February 2020. The current variant that has brought the UK to standstill this Christmas is said to be 70% more...
COVID-19 pandemic has caused a major economic impact all over the globe and has resulted in disruption of “normal” life. Countries across the world are battling to find solutions to this disease that includes strengthening the immune system and...
WHO’s Technical Advisory Group on SARS-CoV-2 Virus Evolution (TAG-VE) was convened on 26th November 2021 to assess the variant B.1.1.529. Based on the available evidences, the group of experts has advised WHO that this variant should be designated as a Variant...
The Lambda variant (lineage C.37) of SARS-CoV-2 was identified in Southern Brazil. This was found to have high prevalence in some south America. In view of high rates of transmissibility across south America, this variant was declared to be a variant...
Results from the phase2 trial support the view that subcutaneous administration of IFN- β for treatment of COVID-19 enhances speed of recovery and reduces mortality.
The extraordinary situation presented by the COVID-19 pandemic has warranted exploring different possible avenues for...
Evidences so far suggest that Omicron variant of SARS-CoV-2 has high transmission rate but fortunately is low on virulence and is not usually leading to severe symptoms of COVID-19 disease or deaths. But existing vaccines seem to be less...
The infection fatality rate (IFR) is more reliable indicator of the extent of the infection. In this study, the researchers found the actual infection rate for COVID-19 in Heinsberg to be five-fold higher than the number officially reported using...
Findings from recently concluded phase 2 clinical trials in Canada and the UK suggest nitric oxide (NO) could be very helpful in preventing and treating COVID-19.
Nitric oxide NO, (not to be confused with nitrous oxide N2O used as anaesthetic in clinical...
There is no clarity on the natural origin of SARS CoV-2 as no intermediate host has been found yet that transmits it from bats to humans. On the other hand, there are circumstantial evidences to suggest a laboratory origin...
The B.1.617 variant that has caused the recent COVID-19 crisis in India has been implicated in increased transmission of the disease among the population and poses a significant challenge with respect to disease severity and effectiveness of currently available...