A new study examined interactions between biomolecules and clay minerals in the soil and shed light on factors that influence trapping of plant-based carbon in the soil. It was found that charge on biomolecules and clay minerals, structure of biomolecules, natural metal constituents in the soil and pairing between biomolecules play key roles in sequestration of carbon in the soil. While presence of positively charged metal ions in the soils favoured carbon trapping, the electrostatic pairing between biomolecules inhibited adsorption of biomolecules to the clay minerals. The findings could be helpful in predicting soil chemistries most effective in trapping carbon in soil which in turn, could pave way for soil-based solutions for reducing carbon in atmosphere and for global warming and climate change.
The carbon cycle involves movement of carbon from atmosphere into plants and animals on the Earth and back into the atmosphere. Ocean, atmosphere and living organisms are main reservoirs or sinks through which carbon cycles. Lot of carbon is stored/sequestrated in rocks, sediments and soils. The dead organisms in rocks and sediments may become fossil fuels over millions of years. Burning of the fossil fuels to meet energy needs release large amount of carbon in the atmosphere which has tipped the atmospheric carbon balance and contributed to global warming and consequent climate change.
Efforts are being made to limit global warming to 1.5°C compared to pre-industrial levels by 2050. To limit global warming to 1.5°C, greenhouse gas emissions must peak before 2025 and be halved by 2030. However, the recent global stocktake has revealed that the world is not on track to limiting temperature rise to 1.5°C by the end of this century. The transition is not fast enough to achieve 43% reduction in greenhouse gas emission by 2030 that could limit global warming within the current ambitions.
It is in this context that the role of soil organic carbon (SOC) in climate change is gaining importance both as a potential source of carbon emission in response to global warming as well as a natural sink of atmospheric carbon.
The historical legacy load of carbon (i.e., emission of about 1,000 billion tons of carbon since 1750 when industrial revolution began) notwithstanding, any increase in global temperature has the potential to release more carbon from soil in the atmosphere hence the imperative to conserve the existing soil carbon stocks.
Soil as a sink of organic carbon
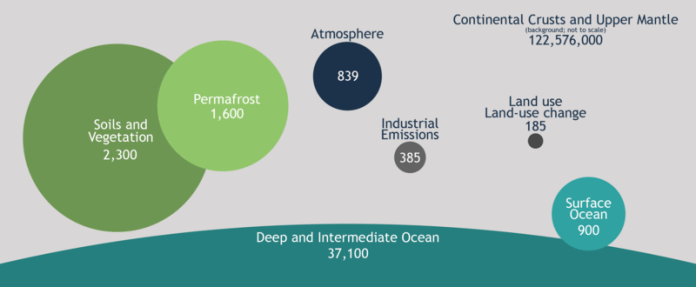
Soil is still Earth’s second largest (after ocean) sink of organic carbon. It holds about 2,500 billion tons of carbon which is about ten times the amount held in the atmosphere, yet it has huge untapped potential to sequester atmospheric carbon. Croplands could trap between 0.90 and 1.85 petagrams (1 Pg = 1015 grams) of carbon (Pg C) per year, which is about 26–53% of the target of the “4 per 1000 Initiative” (that is, 0.4% annual growth rate of the standing global soil organic carbon stocks can offset the current increase in carbon emission in the atmosphere and contribute to meet the climate target). However, the interplay of factors influencing trapping of plant-based organic matter in the soil is not very well understood.
What influences locking of carbon in the soil
A new study sheds light on what determines whether a plant-based organic matter will be trapped when it enters soil or whether it will end up feeding microbes and return carbon to the atmosphere in the form of CO2. Following examination of interactions between biomolecules and clay minerals, the researchers found that charge on biomolecules and clay minerals, structure of biomolecules, natural metal constituents in the soil and pairing between biomolecules play key roles in sequestration of carbon in the soil.
Examination of interactions between clay minerals and individual biomolecules revealed that the binding was predictable. Since clay minerals are negatively charged, biomolecules with positively charged components (lysine, histidine and threonine) experienced strong binding. The binding is also influenced by whether a biomolecule is flexible enough to align its positively charged components with the negatively charged clay minerals.
In addition to electrostatic charge and the structural features of the biomolecules, the natural metal constituents in the soil were found to play an important role in binding through bridge formation. For instance, positively charged magnesium and calcium, formed a bridge between the negatively charged biomolecules and clay minerals to create a bond suggesting natural metal constituents in the soil can facilitate carbon trapping in the soil.
On the other hand, electrostatic attraction between biomolecules themselves impacted the binding adversely. In fact, the energy of attraction between biomolecules was found to be higher than the energy of attraction of a biomolecule to the clay mineral. This meant decreased adsorption of biomolecules to the clay. Thus, while presence of positively charged metal ions in the soils favoured carbon trapping, the electrostatic pairing between biomolecules inhibited adsorption of biomolecules to the clay minerals.
These new findings about how organic carbon biomolecules bind to the clay minerals in the soil could help modify the soil chemistries suitably to favour carbon trapping, thus pave way for soil-based solutions for climate change.
***
References:
- Zomer, R.J., Bossio, D.A., Sommer, R. et al. Global Sequestration Potential of Increased Organic Carbon in Cropland Soils. Sci Rep 7, 15554 (2017). https://doi.org/10.1038/s41598-017-15794-8
- Rumpel, C., Amiraslani, F., Chenu, C. et al. The 4p1000 initiative: Opportunities, limitations and challenges for implementing soil organic carbon sequestration as a sustainable development strategy. Ambio 49, 350–360 (2020). https://doi.org/10.1007/s13280-019-01165-2
- Wang J., Wilson R.S., and Aristilde L., 2024. Electrostatic coupling and water bridging in adsorption hierarchy of biomolecules at water–clay interfaces. PNAS. 8 February 2024.121 (7) e2316569121. DOI: https://doi.org/10.1073/pnas.2316569121
***