A new global network of laboratories for coronaviruses, CoViNet, has been launched by WHO. The aim behind this initiative is to bring together surveillance programs and reference laboratories to support enhanced epidemiological monitoring and laboratory (phenotypic and genotypic) assessment...
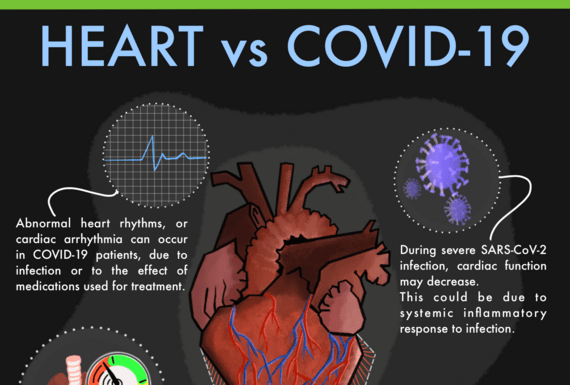
It is known that COVID-19 increases the risk of heart attack, stroke, and Long COVID but what was not known is whether the damage occurs because the virus infects the heart tissue itself, or due to systemic inflammation initiated...
JN.1 sub-variant whose earliest documented sample was reported on 25 August 2023 and which was later reported by the researchers to have higher transmissibility and immune escape ability, has now been designated a variant of interest (VOIs) by WHO.
In the last few...
Spike mutation (S: L455S) is hallmark mutation of JN.1 sub-variant which significantly enhances its immune evasion capability enabling it to effectively evade Class 1 neutralizing antibodies. A study supports use of updated COVID-19 vaccines with spike protein to further...
It is perplexing why China chose to lift zero-COVID policy and do away with the strict NPIs, in winter, just before the Chinese New Year, when a highly transmissible subvariant BF.7 was already in circulation.
“WHO is very concerned over...
Spikevax Bivalent Original/Omicron Booster Vaccine, the first bivalent COVID-19 booster vaccine developed by Moderna has received MHRA approval. Unlike the Spikevax Original, the bivalent version targets both the original coronavirus variants from 2020 and the Omicron variant as well as...
Coronaviruses and influenza viruses are sensitive to acidity of aerosol. pH-mediated rapid inactivation of coronaviruses is possible by enriching the indoor air with non-hazardous levels of nitric acid. Conversely, indoor air filter may unintentionally remove volatile acids thus prolonging...
Cases of co-infections with two variants were earlier reported. Not much was known about viral recombination yielding viruses with hybrid genomes. Two recent studies report cases of genetic recombination among SARS-CoV-2 variants Denta & Omicron. The recombinant, called Deltamicron, had...
WHO has updated its living guidelines on COVID-19 therapeutics. The ninth update released on 03 March 2022 include a conditional recommendation on molnupiravir.
Molnupiravir has become the first oral antiviral drug to be included in the treatment guidelines for COVID-19....
Omicron BA.2 subvariant seems to be more transmissible than BA.1. It also possesses immune-evasive properties that further reduce the protective effect of vaccination against infection.
On 26 November 2021, WHO had designated B.1.1.529 variant of SARS-CoV-2 as a Variant of...
NeoCoV, a coronavirus strain related to MERS-CoV found in bats (NeoCoV is not a new variant of SARS-CoV-2, the human coronavirus strain responsible for COVID-19 pandemic) has been reported to be the first case of a MERS-CoV variant using ACE2....
The search for a universal COVID-19 vaccine, effective against all present and future variants of coronaviruses is an imperative. The idea is to focus on the less-mutating, most conserved region of the virus, instead of the region that frequently mutates....
The government in England recently announced lifting of plan B measures amidst ongoing Covid-19 cases, that makes mask wearing not mandatory, dropping of work from home and no requirement by law of showing of COVID vaccination pass to attend...
Effective 27th January 2022, it will not be mandatory to wear a face covering or need to show COVID pass in England. The measures put in place under Plan B in England, to be lifted.
Earlier on 8th December...
A gene variant of OAS1 has been implicated in reducing the risk of severe COVID-19 disease. This warrants developing agents/drugs that can increase the level of OAS1 enzyme, thereby reducing the severity of COVID-19.
Advanced age and comorbidities are known...
The eighth version (seventh update) of a living guideline is released. It replaces earlier versions. The latest update includes a strong recommendation for the use of baricitinib as an alternative to interleukin-6 (IL-6), a conditional recommendation for the use of...
Deltacron is not a new strain or variant but a case of co-infection with two variants of SARS-CoV-2. Over the past two years, different variants have emerged of the SARS CoV-2 strain with varying degree of infectivity and disease...
A new variant called ‘IHU’ (a new Pangolin lineage named B.1.640.2) is reported to have emerged in south-eastern France.
Researchers in Marseille, France have reported detection of a new variant of novel coronavirus SARS-CoV-2.
The index patient had recent travel history...
Following assessment and approval by the European Medicines Agency (EMA), WHO has issued an emergency use listing (EUL) for Nuvaxovid on 21 December 2021. Earlier on 17 December 2021, the WHO had issued an emergency use listing (EUL) for Covovax.
Covovax and Nuvaxoid thus become the...
Evidences so far suggest that Omicron variant of SARS-CoV-2 has high transmission rate but fortunately is low on virulence and is not usually leading to severe symptoms of COVID-19 disease or deaths. But existing vaccines seem to be less...
Single dose of the vaccine can increase vaccine coverage rapidly which is an imperative in many countries where level of vaccine uptake is not optimal.
WHO has updated its interim recommendations1 on the use of the Janssen Ad26.COV2.S (COVID-19).
One-dose schedule of the...
Sotrovimab, a monoclonal antibody already approved for mild to moderate COVID-19 in several countries gets approval by MHRA in the UK. This antibody was intelligently designed with a mutating virus in mind. A highly conserved region of the spike protein was...
Three adenoviruses used as vectors to produce COVID-19 vaccines, bind to platelet factor 4 (PF4), a protein implicated in the pathogenesis of clotting disorders.
Adenovirus based COVID-19 vaccines such as Oxford/AstraZeneca’s ChAdOx1 use the weakened and genetically modified version of common cold...
One of unusual and most intriguing feature of heavily mutated Omicron variant is that it acquired all the mutations in a single burst in a very short span of time. The degree of change is so much so that some...
In order to raise levels of protection across the population against the Omicron variant, the Joint Committee on Vaccination and Immunisation (JCVI)1 of UK has recommended that the booster programme should be expanded to include all remaining adults aged 18...