A new vaccine, R21/Matrix-M has been recommended by the WHO for the prevention of malaria in children.
Earlier in 2021, WHO had recommended RTS,S/AS01 malaria vaccine for prevention of malaria in children. This was the first malaria vaccine to be recommended.
R21/Matrix-M is the second malaria vaccine recommended by the WHO for prevention of malaria among children.
In view of limited supply of RTS,S/AS01 vaccine, recommendation of the second malaria vaccine R21/Matrix-M is expected to fill the supply gap to meet the high demand.
Recommendation of R21/Matrix-M vaccine was based on positive results of a phase III clinical trial involving 4800 children across five sites in four African countries. The vaccine had a well-tolerated safety profile and offered a high-level efficacy against clinical malaria.
The new vaccine is a low-cost vaccine and is expected to have high public health impact in terms of disease burden in sub-Saharan Africa.
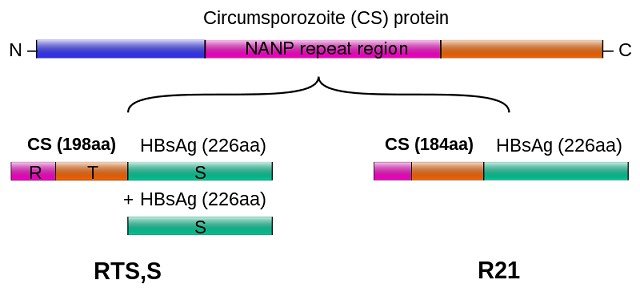
Both R21/Matrix-M and RTS,S/AS01 vaccines are virus-like particle-based vaccines based on circumsporozoite protein (CSP) antigen hence similar. Both target plasmodium sporozoite. However, R21 has a single CSP-hepatitis B surface antigen (HBsAg) fusion protein. This induces higher anti-CSP antibody response and lower anti-HBsAg antibody response which makes it a next-generation RTS,S-like vaccine.
The R21/Matrix-M malaria vaccine is developed by the University of Oxford. It is being manufactured by the Serum Institute of India (SII) which already has production capacity for 100 million doses per year. SII will double the production capacity over the next two years to meet the need.
WHO recommendation paves the way for procurement and purchase of the vaccine for immunisation of children in the endemic regions.
***
Sources:
- WHO News release – WHO recommends R21/Matrix-M vaccine for malaria prevention in updated advice on immunization. Posted on 2 October 2023. Available at https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vaccine-for-malaria-prevention-in-updated-advice-on-immunization Accessed om 3 October 2023.
- Datoo, M. S., et al 2023. A Phase III Randomised Controlled Trial Evaluating the Malaria Vaccine Candidate R21/Matrix-M™ in African Children. Preprint at SSRN. DOI: http://doi.org/10.2139/ssrn.4584076
- Laurens M.B., 2020. RTS,S/AS01 vaccine (Mosquirix™): an overview. Hum Vaccin Immunother. 2020; 16(3): 480–489.Published online 2019 Oct 22. DOI: https://doi.org/10.1080/21645515.2019.1669415
***