Molnupiravir, the world’s first oral drug (approved by MHRA, UK) against COVID-19 along with upcoming drugs such as Paxlovid and sustained vaccination drive has raised hopes that the COVID-19 pandemic may end soon bringing life back to normalcy. Molnupiravir (Lagevrio) is a broad-spectrum drug effective against a host of coronaviruses including VOCs (variants of concern) due to its mechanism of action. The major advantages of these oral drugs are that they reduce cost of intensive care of hospitalization (as they can be taken orally in non-hospitalization settings), thereby reducing burden on the health care system and resources, stop clinical progression of disease to severity if taken timely (within five days of onset of disease) and preventing deaths, and are effective against a wide variety of coronaviruses, including VOCs.
The COVID-19 pandemic has claimed over 5 million lives since March 2020, with over 252 million cases worldwide and incurred an unprecedent financial and economic burden.
Introduction of emergency authorization of vaccines coupled with super massive vaccination drive has considerably reduced the mortality to about 10% of what was seen during pre-vaccination times. However, the pandemic does not seem to be anywhere near the end as evident from the data mentioned in Table I.
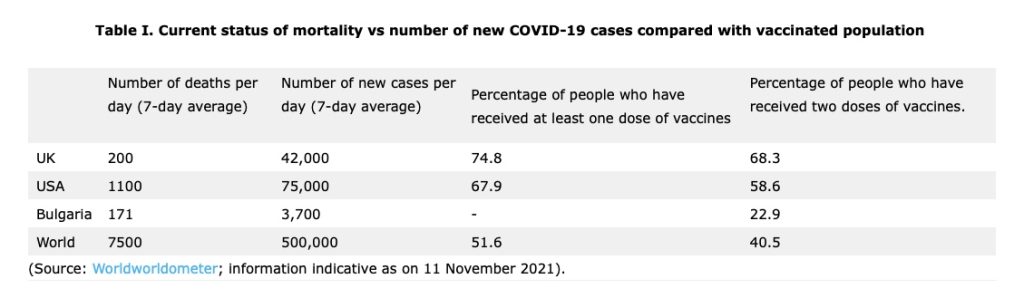
In fact, several countries currently, seem to be in throes of third wave. COVID-19 cases across Europe have started reaching record levels, making the region the epicentre of the pandemic. In the past couple of weeks, Europe and Central Asia witnessed a 6% increase and 12% increase respectively, in the number of COVID-19 cases. Over the last month, the region has faced a greater than 55% increase in new COVID-19 cases accounting for 59% of all cases globally and 48% of reported deaths.1 The situation in Central and Eastern European countries like Romania, Bulgaria, Ukraine etc. is further complicated due to low vaccination uptakes compared to Western Europe.
The situation in the USA is far from being satisfactory. In China, there are media reports of ring-fencing of Beijing as precaution against outbreaks in several provinces within the country. Notwithstanding the level of vaccination achieved, if these current trends are any indication, there does not seem to be any guarantee, that the rest of the regions of the world will not see a situation similar to what we see in Europe and Central Asia currently, in the near foreseeable future.
With this background, the recent announcements of the encouraging results of clinical trials for the two new antiviral pills (Merck’s Molnupiravir and Pfizer’s Paxlovid) against COVID-19 and subsequent quick approval of Molnupiravir in the UK is gaining significance as a new orally available second line of protection (after vaccination) for recently diagnosed cases against progression of the disease symptoms, thereby preventing requirements of hospitalisation or even death.
Current approaches to deal with the pandemic
Coronaviruses display remarkably high rates of errors during replication (due to lack of proofreading nuclease activity of their polymerases) which remain uncorrected and accumulate to act as source of variation. More the transmission, more the replication errors and more accumulating mutations in the genome, leading to evolution of new variants. Hence, social restrictions to limit transmission is important for prevention of new cases as well as for prevention of evolution of new variants. By far, vaccination has shown great promise in the prevention of disease symptoms and progression to severity, requiring hospitalisation. In countries with high vaccination rates e.g., the UK, mortality rates are down to about 10% of what was seen during earlier waves. Yet, a good number of people are requiring hospitalisation.
For mild to severe cases, various approaches have been tried. Moderately severe cases require oxygen support, while severe cases require intubation with intensive care. Dexamethasone is found to be most-cost effective in severe cases of hospitalisation. The antiviral, remdesivir seems to be effective but costly, and hence is unlikely to be a cost-effective treatment for COVID-192.
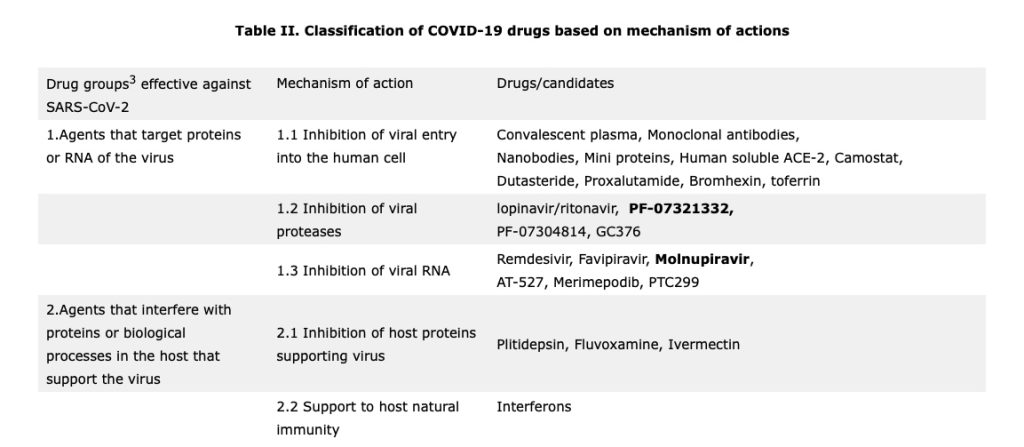
Antivirals for COVID-19 fall into three groups (refer to point no. 1 in Table II above). The first group consists of drugs like Umifenovir (currently used for the treatment of influenza in Russia and China) inhibit entry of virus into the human cells while the second group comprises of viral RNA inhibitors like Remdesivir, Favipiravir and Molnupiravir act as competitive nucleoside analogue to cause multiple non-sense mutations (RNA mutagenesis), thereby interfering with viral replication. The third group is of viral protease inhibitors like lopinavir/ritonavir, PF-07321332, and PF-07304814 which block viral protease enzyme thereby disabling viruses to make new viruses, thus reducing viral load.
Despite several previous episodes of epidemics of influenzas and two recent outbreaks of coronavirus (2003 outbreak in China attributed to SARS-CoV and MERS outbreak of 2012), only one antiviral drug (Remdesivir) had seen the light of the day and could be of some help in the current pandemic, albeit it was originally developed to treat Hepatitis C and Ebola. Remdesivir was helpful in treating COVID-19 patients with severe symptoms in hospital settings, but is highly costly and therefore does not render an affordable cost-effective treatment.
The need of the hour are drugs that could stop clinical progression of the new cases of COVID-19 from no symptoms to mild to moderate or severe, thereby minimising need of hospitalisation and preventing COVID related deaths.
Molnupiravir and PF-07321332, the two anti-viral drugs show promise in stopping clinical progression of asymptomatic or mild cases
Coronaviruses use an RNA-dependent RNA polymerase (RdRp) for the replication and transcription of their RNA genome which makes RdRp is an important target for the antiviral drugs against coronaviruses4.
Molnupiravir, an inhibitor of viral RNA polymerase, a competitive nucleoside analogue in viral RNA dependent RNA polymerase, causing multiple non-sense mutations induces RNA mutagenesis. It increases the frequency of viral RNA mutations and impairs SARS-CoV-2 replication. It inhibits viral replication by a mechanism known as ‘lethal mutagenesis’. Molnupiravir disrupts the fidelity of SARS-CoV-2 genome replication and prevents viral propagation by fostering error accumulation in a process referred to as ‘error catastrophe’ 4,5.
Molnupiravir, developed by Ridgeback therapeutics and MSD (Merck) as trade name Lagevrio, is a prodrug of ß-D-N4-hydroxycytidine and has been shown to decrease viral replication 100,000-fold in mice engineered to have human lung tissue6. In case of ferrets, molnupiravir not only reduced symptoms, but also led to zero virus transmission within 24 hours6. Molnupiravir was well tolerated with no significant adverse events in a randomized, double-blind, placebo-controlled, First-in-Human study designed to evaluate the safety, tolerability, and pharmacokinetics of the drug, following oral administration to healthy volunteers in a total of 130 subjects7,8. In phase 2/3 clinical trials, Lagevrio was found to be to be effective in reducing the risk of hospitalisation or death for at-risk non-hospitalised adults with mild to moderate COVID-19 by 50%9. Lagevrio is thus the world’s first approved anti-viral drug that can be taken orally rather than intravenously. This is important as it can be administered in a non-hospital setting, before COVID-19 has progressed to a severe stage. It should be taken as soon as possible following a positive COVID-19 test and within five days of symptoms onset. However, it cannot be regarded as a substitute for vaccination, hence the vaccination drive should continue.
Paxlovid (PF-07321332) on the other hand, acts by way of inhibition of viral protease SARS-CoV-2-3CL protease, an enzyme that the coronavirus needs to replicate. It is used either alone or in combination with low dose ritonavir.
Ritonavir is an HIV protease inhibitor, is commonly administered with other protease inhibitors as part of highly active antiretroviral therapy for HIV, as it inhibits hepatic metabolism of the partner drug.
Based on an interim analysis of the Phase 2/3 EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients)10 randomized, double-blind study of non-hospitalized adult patients with COVID-19, who are at high risk of progressing to severe illness, Paxlovid showed an 89% reduction in risk of COVID-19-related hospitalization or death compared to placebo in patients treated within three days of symptom onset. The adverse events associated with Paxlovid were comparable to those of placebo and very mild in intensity.
Another advantage of Paxlovid is that it demonstrated potent antiviral in vitro activity against circulating variants of concern (VOCs), as well as other known coronaviruses. Paxlovid thus has potential to be used as a therapeutic for multiple types of coronavirus infections.
It won’t be long before we see the approval of Paxlovid as well as a therapeutic agent in fight against COVID-19.
While Molnupiravir is a nucleoside analogue that interferes with viral RNA replication, Paxlovid is an inhibitor of 3CL protease, an enzyme required for coronavirus replication.
The main questions raised for both these oral anti-viral drugs will revolve around their effectiveness, safety, whether or not they will work against existing and upcoming variants, development of resistance to these drugs and their accessibility to poorer countries11. While both Molnupiravir and Paxlovid fare well in answers to first three questions, it will be important to analyse people that don’t respond to either of the drugs to rule out viral resistance and also to monitor people having a weak immune system and are administered these drugs for COVID-19 treatment. Apart from viral resistance, the accessibility of these drugs to Third World countries will pose a major threat to reduce the pandemic as these countries may not be able to afford the same, e.g, Molnupiravir treatment costs USD 700 per patient while that of Paxlovid remains to be seen but may be in the same ball park. Another challenge could that the richer and affluent countries may start hoarding the doses for their own population, making access difficult to all. Even if one makes the drug (molnupiravir) available to poorer countries, they might not have the diagnostic capacity to treat patients with molnupiravir early in the course of their illness, when treatment could be most effective12.
Nevertheless, these two novel anti-viral drugs seem to have a huge potential in treatment of COVID-19 and may help hasten the end of the pandemic soon, leaving COVID-19 as an endemic disease with minor effects.
***
References:
- WHO Europe 2021. Statement – Update on COVID-19: Europe and central Asia again at the epicentre of the pandemic. Posted 4 November 2021. Available online here
- Congly, S.E., Varughese, R.A., Brown, C.E. et al. Treatment of moderate to severe respiratory COVID-19: a cost-utility analysis. Sci Rep 11, 17787 (2021). https://doi.org/10.1038/s41598-021-97259-7
- Şimşek-Yavuz S, Komsuoğlu Çelikyurt FI. Antiviral treatment of COVID-19: An update. Turk J Med Sci. 2021 Aug 15. DOI: https://doi.org/10.3906/sag-2106-250
- Kabinger, F., Stiller, C., Schmitzová, J. et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol 28, 740–746 (2021). Published: 11 August 2021. DOI: https://doi.org/10.1038/s41594-021-00651-0
- Malone, B., Campbell, E.A. Molnupiravir: coding for catastrophe. Nat Struct Mol Biol 28, 706–708 (2021). Published: 13 September 2021. DOI: https://doi.org/10.1038/s41594-021-00657-8
- Soni R. 2021. Molnupiravir: A Game Changing Oral Pill for Treatment of COVID-19. Scientific European. Published 5 May 2021. Available online at http://scientificeuropean.co.uk/covid-19/molnupiravir-a-game-changing-oral-pill-for-treatment-of-covid-19/
- Painter W., Holman W., et al 2021. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity against SARS-CoV-2. Antimicrobial Agents and Chemotherapy. Published online April 19, 2021. DOI: https://doi.org/10.1128/AAC.02428-20
- ClinicalTrial.gov 2021. A Randomized, Double-Blind, Placebo-Controlled, First-in-Human Study Designed to Evaluate the Safety, Tolerability, and Pharmacokinetics of EIDD-2801 Following Oral Administration to Healthy Volunteers. Sponsor: Ridgeback Biotherapeutics, LP. ClinicalTrials.gov Identifier: NCT04392219. Available online at https://clinicaltrials.gov/ct2/show/NCT04392219?term=NCT04392219&draw=2&rank=1 Accessed on 20 April 2021.
- UK Govt 2021. Press release – First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA. Published 4 November 2021. Available online at https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra
- Pfizer 2021. News – Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death By 89% In Interim Analysis of Phase 2/3 EPIC-HR Study. Posted November 05, 2021. Available online here
- Ledford H., 2021. COVID antiviral pills: what scientists still want to know. Nature News Explainer. Published 10 November 2021. DOI: https://doi.org/10.1038/d41586-021-03074-5
- Willyard C., 2021. How antiviral pill molnupiravir shot ahead in the COVID drug hunt. Nature News. Published 08 October 2021. DOI: https://doi.org/10.1038/d41586-021-02783-1
***