Sun Pharma has presented data on ODOMZO® (drug for treatment of skin cancer) and LEVULAN® KERASTICK® + BLU-U®, (for treating precancerous lesions) supporting safety and efficacy.
ODOMZO®
ODOMZO® (Sonidegib) was approved by FDA in July 2015. This was acquired by Sun...
A novel study based on nanotechnology generates hope for treating acute kidney injury and failure.
Kidney is an essential vital organ which performs critical functions in the body. It removes wastes and extra water from our blood stream to produce...
A set of studies describe a human antibody which can effectively prevent the deadliest malaria caused by the parasite Plasmodium falciparum
Malaria is one of the most severe public health problems worldwide. It is a life-threatening disease caused by parasites...
The ketogenic diet (low carbohydrate, limited protein and high fat) shows improved effectiveness of a new class of cancer drugs in cancer treatment
Cancer treatment has been at the forefront of the medical and research community worldwide. 100 percent successful...
Scientists have shown that environmental stress can affect normal development of nervous system in worms who are approaching puberty
Scientists have been trying to understand how our genes (our genetic makeup) and different environmental factors shape our nervous system during...
Early nerve transfer surgery to treat paralysis of arms and hands due to spinal injury is helpful in improving function. Post two years of surgery and physiotherapy, patients regained function in elbows and hands leading to improvement in independence...
Scientists have found a new way in mice to get relief from the chronic neuropathic pain
Neuropathic pain in humans is a chronic pain associated with nerve damage like neuropathy. This is very difficult to treat chronic type of pain...
Research has shown that another protein called tau is responsible for early symptoms of Alzheimer’s disease and this information may aid in developing therapies.
Alzheimer’s Disease (AD) or simply Alzheimer’s has no cure and it also cannot be prevented. Deferring...
New study shows a method to individually distinguish cells in the body in order to advance precision medicine or personalised therapeutic treatments.
Precision medicine is a new model of healthcare in which genetic data, microbiome data and overall information on...
Researchers have created 2-dimensional mineral nanoparticles to deliver treatment in the body for cartilage regeneration
Osteoarthritis is a degenerative disease which affects 630 million people worldwide which is almost 15 percent of the entire population on the planet. In osteoarthritis,...
Researchers have designed a novel HIV drug which can help fight advanced, drug-resistant HIV infection in patients who have no other treatment options.
At least 40 million people are living with HIV till mid-2018. HIV (Human Immunodeficiency Virus) is a...
A newly discovered antibiotic follows a unique mechanism in fighting drug-resistant bacteria responsible for UTIs.
Antibiotic resistance is a major global threat to healthcare. Antibiotic resistance occurs when bacteria modify themselves in some manner which then either reduces or completely...
A breakthrough study has shown a way forward to create medicines/drugs which have fewer unwanted side effects than we have today
Medicines in today’s times comes from a variety of sources. Side effect in medication is a big problem. Unwanted...
Study describes a novel mechanism involved in progression of non-alcoholic fatty liver disease and highlights protein Mitofusin 2 as having the potential to be a possible treatment model
Non-alcoholic fatty liver disease is the most common liver condition which affects...
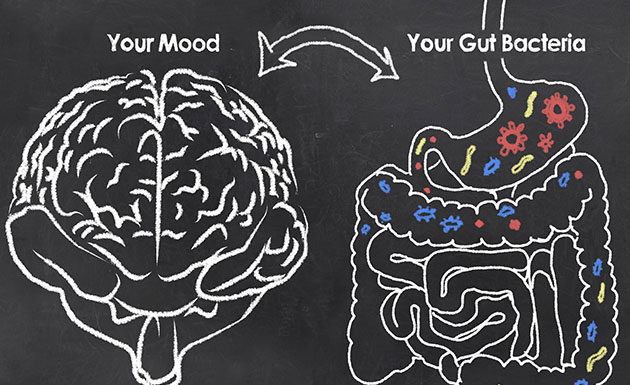
Scientists have identified several groups of bacteria which varied along with depression and quality of life in humans
Our gastrointestinal (GI) track has a trillion of microorganisms. The microbes which reside in our gut perform important functions and are thought...
Fibrotic diseases are known to affect several vital organs in the body and are a major cause of mortality and morbidity. There has been a little success in treatment of these diseases so far. ILB®, the Low Molecular Weight...
A unique immunotherapy-based antibody approach has been developed which targets cancers consisting of solid tumours.
Ovarian cancer is the seventh most common cancer in women globally. Ovaries are the two reproductive glands that produce eggs in a female and also...
Study had shown recovery from paralysis using a novel method of neurotechnology
The vertebrae in our body are bones which make up the spine. Our spine contains several nerves which extend from our brain down till the lower back. Our...
An important marker for diabetes development has been identified.
The two important hormones produced in the pancreas – glucagon and insulin – control proper glucose levels in response to the food that we consume. Glucagon increases Hepatic Glucose Production (HGP)...
New study in mice shows that getting enough sleep every night could provide protection from cardiovascular diseases
Getting enough sleep is a general advice given by doctors as it is associated with maintaining good health. When someone gets adequate sleep,...
Research shows that neutralizing antibodies which are induced by vaccination can protect animals from HIV infection.
Developing a safe and effective HIV (Human Immunodeficiency Virus) vaccine, despite up to 30 ongoing clinical trials, is a challenge faced by research community...
Study shows that a person’s body weight influences the effects of low-dose aspirin in preventing cardiovascular events
Daily aspirin therapy according to body weight
Studies published in The Lancet has shown in a randomized trial that effects of common medicine aspirin in preventing...
Scientists have identified a distinct nerve-signalling pathway which could help to recover from sustained pain after an injury.
We all know pain – the unpleasant feeling caused by a burn or ache or headache. Any kind of pain in our...
A novel treatment which “prevents” oesophageal cancer in at-risk patients is reported in a large clinical trial.
Oesophageal cancer is the eight most common cancer worldwide and one of the most dangerous. This type of cancer starts in the oesophagus...
A study suggests that both excessive consumption of alcohol and total abstinence contribute to a person’s risk of developing dementia later in life
Dementia is group of brain disorders which affect a person’s mental cognitive tasks like memory, performance, concentration,...